2022-11-15T08:01:00
(BPT) – Narcolepsy is often dismissed as a minor sleep issue, but in reality, it’s a serious, chronic sleep disorder that can negatively impact overall health and increases odds of having heart disease, diabetes, obesity, high blood pressure and a stroke. What’s more, a study that compared 9,312 adults with narcolepsy to 46,559 similar adults who do not live with the sleep disorder showed that people with narcolepsy have 1.6x greater odds of having a heart attack.
Since studies show narcolepsy is associated with higher rates of heart diseases, people with narcolepsy should work with their doctor to establish an overall health plan, which may include strategies to limit the impact of cardiovascular risk factors.
“Making heart-healthy choices is critical for people with narcolepsy due to their increased risk for cardiovascular problems,” said Rick Bogan, MD, FCCP, FAASM, founder of Bogan Sleep Consultants and associate clinical professor at University of South Carolina School of Medicine. “Lifestyle adjustments like watching salt intake, not smoking and exercising can play an important role in helping people with narcolepsy better manage their cardiovascular health.”
Narcolepsy is a debilitating neurological disorder characterized by the inability to regulate sleep-wake cycles normally, affecting an estimated one in 2,000 people in the U.S. Everyone with narcolepsy has excessive daytime sleepiness symptoms, meaning they are overly tired during the day, regardless of how much they slept the night before. Of those affected by narcolepsy, about 70% are believed to have cataplexy, which is a sudden, brief loss of muscle control triggered by strong emotions like embarrassment, laughter, surprise or anger.
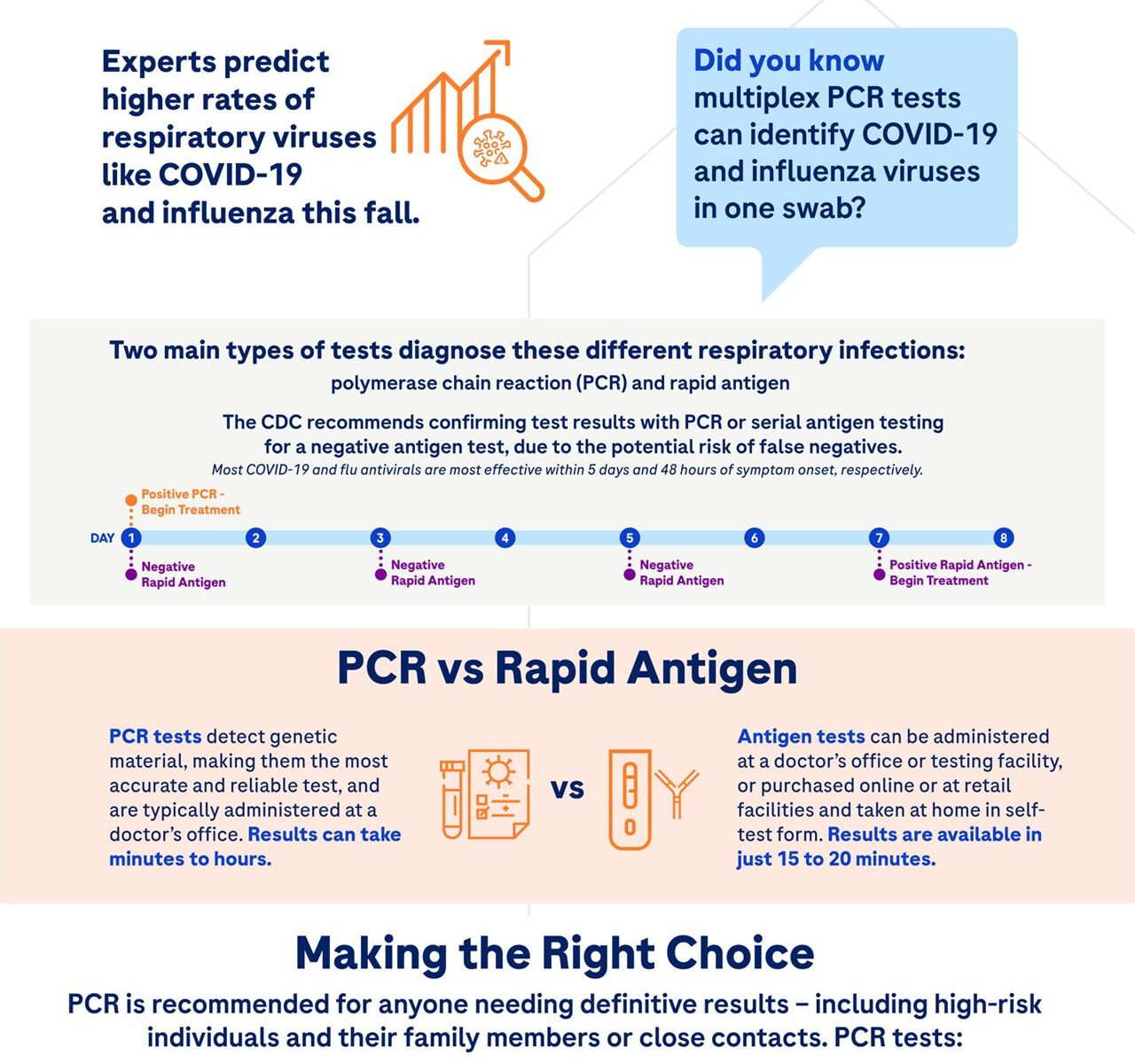
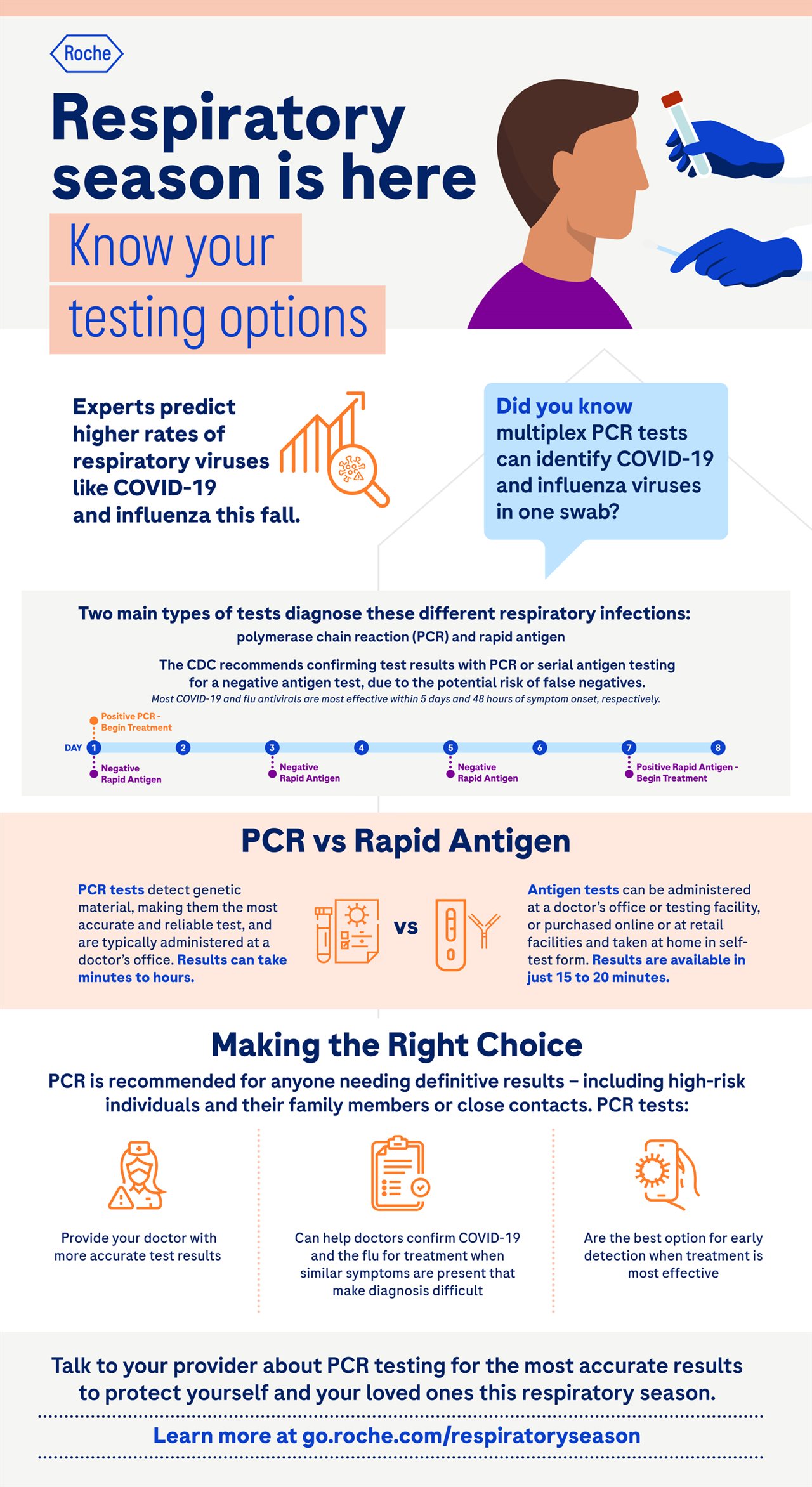
When it comes to risk factors for cardiovascular issues among patients with narcolepsy, Dr. Bogan emphasizes the importance of lifestyle changes, such as watching salt consumption. Many people know that sodium intake is important but may not be aware of its connection to heart health. For the general population, the American Heart Association recommends an ideal limit of 1,500 mg of sodium a day for most adults, but a study from the Centers for Disease Control and Prevention found Americans 2 and older consume an average of 3,409 mg of sodium a day. For most adults, lowering sodium intake by 1,000 mg per day can help reduce the risk for high blood pressure, heart disease and stroke. For patients with narcolepsy, healthy eating and food preparation can help them consume less salt, but many patients don’t realize that some medications may also contain significant amounts of sodium.
Xyrem (sodium oxybate) oral solution is approved to treat people 7 years or older with narcolepsy who have excessive daytime sleepiness and cataplexy; however, this high-sodium oxybate can contain more than the recommended daily amount of sodium. This high-sodium oxybate can contain as much as 1,640 mg of sodium per day at the maximum recommended nightly dose of 9 grams. At the recommended maximum dose, that’s more sodium than four orders of large fries (based on an average of 380 mg of sodium per one large serving), which can contain approximately 1,520 mg of sodium according to a 2012 USDA analysis of three fast food chains.
With 92% less sodium than high-sodium oxybate, XYWAV (calcium, magnesium, potassium, and sodium oxybates) oral solution is the first and only lower-sodium oxybate treatment approved by the U.S. Food and Drug Administration for people seven years or older to treat excessive daytime sleepiness and cataplexy in narcolepsy. Collaborate with your doctor to determine a customized plan to help manage your narcolepsy symptoms and overall health.
XYWAV may cause serious side effects including Central Nervous System (CNS) depression, abuse and misuse, breathing problems, mental health problems and sleepwalking. Because of the risk of CNS depression, abuse and misuse, XYWAV is available only by prescription, and filled through the central pharmacy in the XYWAV and XYREM REMS. Please see below for more Important Safety Information on XYWAV.
To learn more about XYWAV, a lower-sodium oxybate, as a treatment for narcolepsy symptoms of excessive daytime sleepiness and cataplexy, visit XYWAV.com/narcolepsy.
About Xywav® (calcium, magnesium, potassium, and sodium oxybates) oral solution
Xywav is a lower-sodium oxybate approved by the U.S. Food and Drug Administration (FDA) for the treatment of cataplexy or excessive daytime sleepiness in patients 7 years of age and older with narcolepsy. FDA recognized seven years of Orphan Drug Exclusivity for Xywav in June 2021 for the treatment of cataplexy or excessive daytime sleepiness in patients 7 years of age and older with narcolepsy. The Office of Orphan Product Development (OOPD) at FDA found Xywav to be clinically superior to Xyrem by means of greater cardiovascular safety because Xywav provides a greatly reduced chronic sodium burden compared to Xyrem. According to FDA, these differences in sodium content will be clinically meaningful in reducing cardiovascular disease for many people with narcolepsy who suffer from EDS and/or cataplexy. There are no head-to-head data for Xywav and Xyrem. Xywav is comprised of a unique composition of cations resulting in 92% less sodium. At the recommended dosage range of 6 to 9 grams, that is a reduction of approximately 1,000 to 1,500 mg of sodium per night. While the exact mechanism of action of Xywav is unknown, it is hypothesized that the therapeutic effects of Xywav on cataplexy and excessive daytime sleepiness are thought to work during sleep to help with symptoms during the day. Because of the risks of CNS depression and abuse and misuse, Xywav is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the XYWAV and XYREM REMS.
Important Safety Information
WARNING: Taking XYWAV with other central nervous system (CNS) depressants such as medicines used to make you or your child fall asleep, including opioid analgesics, benzodiazepines, sedating antidepressants, antipsychotics, sedating anti-epileptic medicines, general anesthetics, muscle relaxants, alcohol, or street drugs, may cause serious medical problems, including trouble breathing (respiratory depression), low blood pressure (hypotension), changes in alertness (drowsiness), fainting (syncope), and death.
The active ingredient of XYWAV is a form of gamma hydroxybutyrate (GHB). Abuse or misuse of illegal GHB alone or with other drugs that cause changes in alertness (or consciousness) has caused serious side effects. These effects include seizures, trouble breathing (respiratory depression), changes in alertness (drowsiness), coma, and death. Call your doctor right away if you or your child has any of these serious side effects.
Because of these risks, you have to go through the XYWAV and XYREM REMS to have your or your child’s prescription for XYWAV filled.
Do not take XYWAV if you take or your child takes other sleep medicines or sedatives (medicines that cause sleepiness), drinks alcohol, or has a rare problem called succinic semialdehyde dehydrogenase deficiency.
Keep XYWAV in a safe place to prevent abuse and misuse. Selling or giving away XYWAV may harm others and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
Anyone who takes XYWAV should not do anything that requires them to be fully awake or is dangerous, including driving a car, using heavy machinery, or flying an airplane, for at least 6 hours after taking XYWAV. Those activities should not be done until you know how XYWAV affects you or your child.
XYWAV can cause serious side effects, including the following:
- Breathing problems, including slower breathing, trouble breathing, and/or short periods of not breathing while sleeping (sleep apnea). People who already have breathing or lung problems have a higher chance of having breathing problems when they use XYWAV.
- Mental health problems, including confusion, seeing or hearing things that are not real (hallucinations), unusual or disturbing thoughts (abnormal thinking), feeling anxious or upset, depression, thoughts of killing yourself or trying to kill yourself, increased tiredness, feelings of guilt or worthlessness, or difficulty concentrating. Tell your doctor if you or your child have or had depression or have tried to harm yourself or themselves. Call your doctor right away if you have or your child has symptoms of mental health problems or a change in weight or appetite.
- Sleepwalking. Sleepwalking can cause injuries. Call your doctor if this occurs.
The most common side effects of XYWAV in adults include nausea, headache, dizziness, anxiety, insomnia, decreased appetite, excessive sweating (hyperhidrosis), vomiting, diarrhea, dry mouth, parasomnia (a sleep disorder that can include abnormal dreams, abnormal rapid eye movement (REM) sleep, sleep paralysis, sleep talking, sleep terror, sleep-related eating disorder, sleep walking, and other abnormal sleep-related events), somnolence, fatigue, and tremor.
The most common side effects of XYREM (which also contains oxybate like XYWAV) in children include nausea, bedwetting, vomiting, headache, weight decrease, decreased appetite, dizziness, and sleepwalking.
XYWAV can cause physical dependence and craving for the medicine when it is not taken as directed. These are not all the possible side effects of XYWAV.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see full Prescribing Information, including BOXED Warning, and Medication Guide here: https://pp.jazzpharma.com/pi/xywav.en.USPI.pdf.