2024-12-11T15:05:00
(BPT) – Sponsored by Boston Scientific
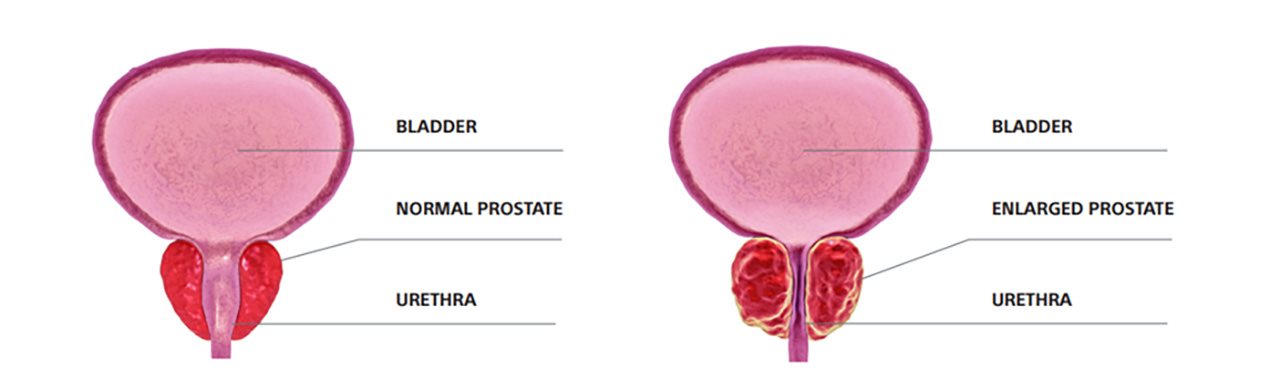
Enlarged prostate, also known as benign prostatic hyperplasia (BPH), is a common condition that affects about 50% of men by age 60 and up to 90% of men by age 85.1 As men age, the prostate can grow from the size of a walnut to about the size of a lemon, placing pressure on the urethra and obstructing the flow of urine.1 While enlarged prostate (BPH) can be a burdensome condition, it is non-cancerous and there are several treatment options available.2
What are the early signs of enlarged prostate and common symptoms?
Symptoms of enlarged prostate vary and tend to worsen over time. Common symptoms of enlarged prostate include frequent and urgent urination, trouble starting a urine stream, urinary retention, urinary incontinence and more.2 Interestingly, the size of the prostate doesn’t always determine how serious symptoms are. For instance, some men with slightly enlarged prostates can experience major symptoms, while others with very enlarged prostates can experience minor symptoms.3 Less frequent but serious symptoms to watch out for include urinary tract infection, not being able to pee and blood in the urine.3
Is there a way to treat enlarged prostate? What are some common treatment options?
It is important to discuss treatment options with a health care provider as soon as symptoms begin.3 While there isn’t a cure for enlarged prostate, a range of treatment approaches can help alleviate symptoms, including:
- Watch and wait: Closely monitoring the condition is the first consideration for most men presenting with early signs of enlarged prostate. Some men will also turn to behavioral modifications such as drinking less caffeine to avoid worsening symptoms.4
- Medication: Another way of treating enlarged prostate is through commonly prescribed medications that can relax the tension on your urethra making it easier to urinate. Other medications block hormones, which in turn can slow the growth of the prostate gland.5
- Surgery: In instances where medications are ineffective or patients wish to avoid daily pills and their associated side effects, symptoms are severe or complications arise, health care providers may recommend surgery to remove the tissue around the urethra or widen the urethra itself.5
Are there alternatives to surgery?
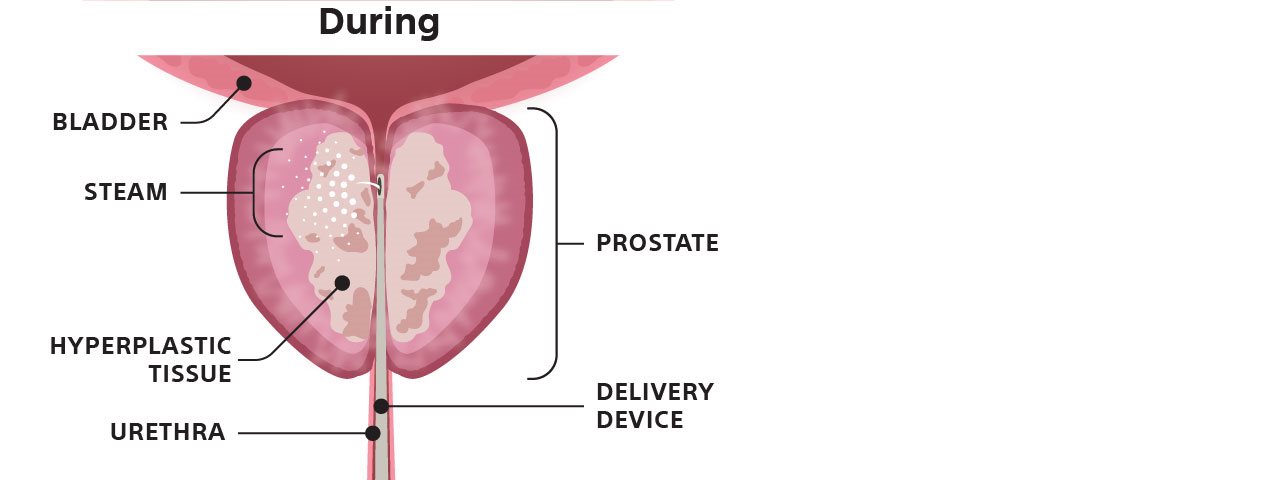
For those who want to avoid surgery, there are minimally-invasive treatment options, like Rezūm™ Water Vapor Therapy — a short, in-office procedure requiring no general anesthesia.6 The procedure uses water vapor, or steam, to remove obstructive prostate tissue over time — helping reduce the symptoms of BPH.7
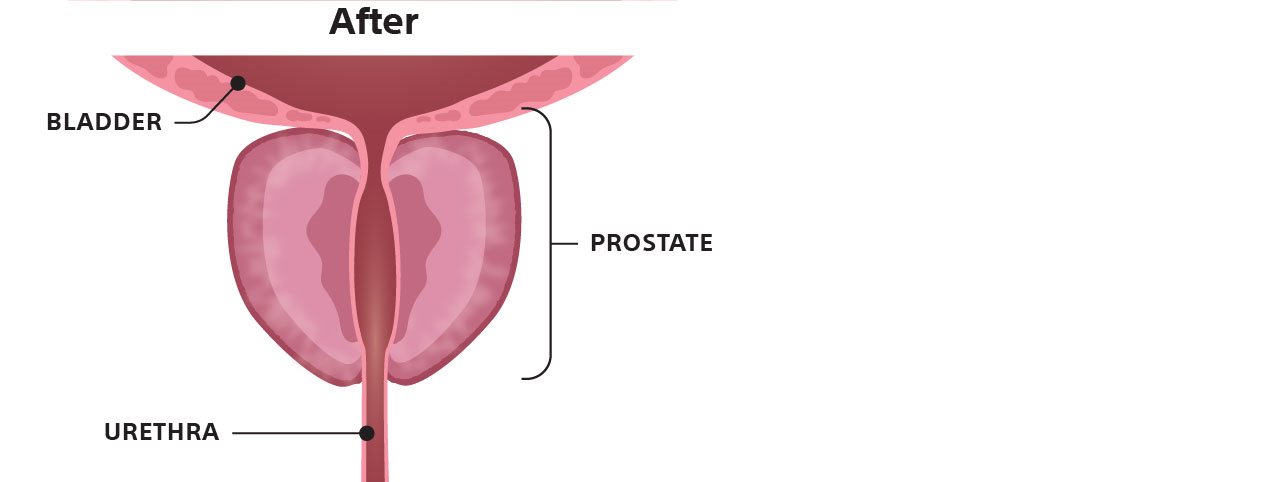
Most patients return to their regular activities within a few days and see improvement in their symptoms over a few weeks. Research has also shown that Rezūm provides patients with significant and sustained BPH symptom relief through 5 years.6
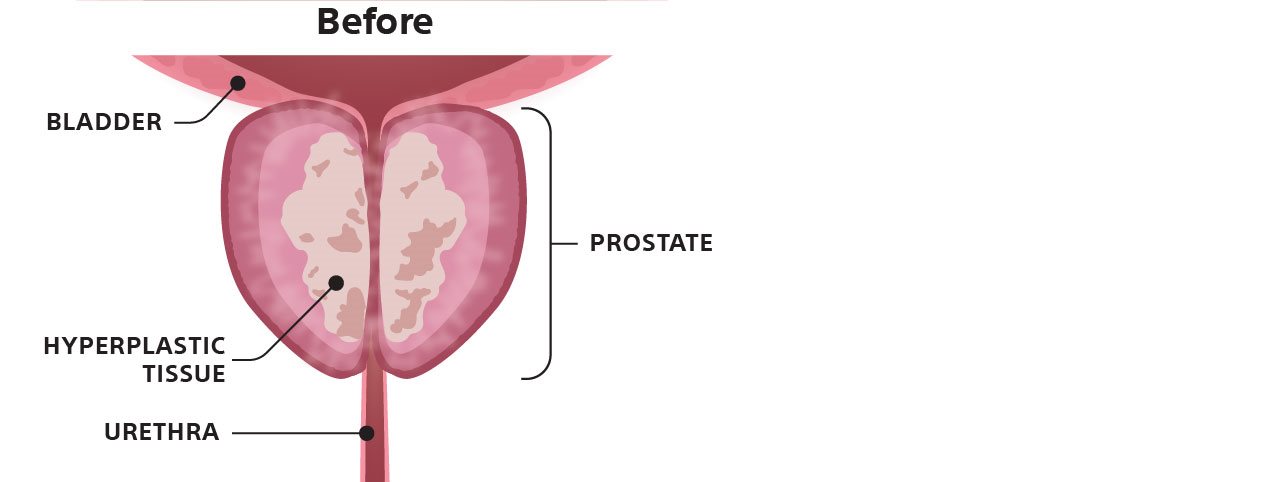
Graphic depicts how Rezūm Water Vapor Therapy affects the prostate.
To learn more about Rezūm Water Vapor Therapy and to find a doctor near you, please visit https://www.rezum.com/find-a-doctor.html/.
As with any medical treatment, there are some risks which include painful or frequent urination, blood in the urine or semen, decrease in ejaculatory volume, urinary tract infection (UTI), inability to urinate or completely empty the bladder and urgent need to urinate. Visit Rezum.com for a complete list of risks and talk to your doctor about the benefits and risks associated with Rezūm.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
All treatments have inherent and associated risks. The Rezūm System is intended to relieve symptoms, obstructions and reduce prostate tissue associated with BPH. It is indicated for men ≥ 50 years of age with a prostate volume 30cm3 ≤ 80cm3. The Rezūm System is also indicated for treatment of prostate with hyperplasia of the central zone and/or a median lobe. Potential risks include but are not limited to painful urination (dysuria), blood in the urine (hematuria), blood in the semen (hematospermia), decrease in ejaculatory volume, suspected urinary tract infection (UTI), and urinary frequency, retention or urgency. You should talk with your doctor about benefits and risks before moving forward with any treatment option.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.
References
1. Harvard Health. The growing problem of an enlarged prostate gland. Available at: https://www.health.harvard.edu/mens-health/the-growing-problem-of-an-enlarged-prostate-gland. Accessed December 2024.
2. Health Information. Urologic Diseases. Prostate Problems. Prostate Enlargement (Benign Prostatic Hyperplasia). National Institute of Diabetes and Digestive and Kidney Diseases. Available at: https://www.niddk.nih.gov/health-information/urologic-diseases/prostate-problems/prostate-enlargement-benign-prostatic-hyperplasia#benign. Accessed December 2024.
3. Diseases & Conditions. Symptoms & causes. Benign prostatic hyperplasia (BPH). Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/symptoms-causes/syc-20370087. Accessed December 2024.
4. UpToDate. Patient education: Benign prostatic hyperplasia (BPH) (Beyond the Basics). Available at: https://www.uptodate.com/contents/benign-prostatic-hyperplasia-bph-beyond-the-basics. Accessed December 2024.
5. Diseases & Conditions. Diagnosis & treatment. Benign prostatic hyperplasia (BPH). Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/diagnosis-treatment/drc-20370093. Accessed December 2024.
6. McVary KT, Gittelman MC, Goldberg KA, et al. Final 5-year outcomes of the multicenter randomized sham-controlled trial of Rezūm water vapor thermal therapy for treatment of moderate-to-severe lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2021 Sep;206(3):715-24.
7. McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation: A multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016 May;195(5):1529-38.
Get the Facts about Enlarged Prostate
(BPT) – Sponsored by Boston Scientific
Enlarged prostate, also known as benign prostatic hyperplasia (BPH), is a common condition that affects about 50% of men by age 60 and up to 90% of men by age 85. 1 As men age, the prostate can grow from the size of a walnut to about the size of a lemon, placing pressure on the urethra and obstructing the flow of urine. 1 While enlarged prostate (BPH) can be a burdensome condition, it is non-cancerous and there are several treatment options available. 2
What are the early signs of enlarged prostate and common symptoms?
Symptoms of enlarged prostate vary and tend to worsen over time. Common symptoms of enlarged prostate include frequent and urgent urination, trouble starting a urine stream, urinary retention, urinary incontinence and more. 2 Interestingly, the size of the prostate doesn’t always determine how serious symptoms are. For instance, some men with slightly enlarged prostates can experience major symptoms, while others with very enlarged prostates can experience minor symptoms. 3 Less frequent but serious symptoms to watch out for include urinary tract infection, not being able to pee and blood in the urine. 3
Is there a way to treat enlarged prostate? What are some common treatment options?
It is important to discuss treatment options with a health care provider as soon as symptoms begin. 3 While there isn’t a cure for enlarged prostate, a range of treatment approaches can help alleviate symptoms, including:
• Watch and wait: Closely monitoring the condition is the first consideration for most men presenting with early signs of enlarged prostate. Some men will also turn to behavioral modifications such as drinking less caffeine to avoid worsening symptoms. 4
• Medication: Another way of treating enlarged prostate is through commonly prescribed medications that can relax the tension on your urethra making it easier to urinate. Other medications block hormones, which in turn can slow the growth of the prostate gland. 5
• Surgery: In instances where medications are ineffective or patients wish to avoid daily pills and their associated side effects, symptoms are severe or complications arise, health care providers may recommend surgery to remove the tissue around the urethra or widen the urethra itself. 5
Are there alternatives to surgery?
For those who want to avoid surgery, there are minimally-invasive treatment options, like Rezūm™ Water Vapor Therapy — a short, in-office procedure requiring no general anesthesia. 6 The procedure uses water vapor, or steam, to remove obstructive prostate tissue over time — helping reduce the symptoms of BPH. 7
Most patients return to their regular activities within a few days and see improvement in their symptoms over a few weeks. Research has also shown that Rezūm provides patients with significant and sustained BPH symptom relief through 5 years. 6
Graphic depicts how Rezūm Water Vapor Therapy affects the prostate.
To learn more about Rezūm Water Vapor Therapy and to find a doctor near you, please visit https://www.rezum.com/find-a-doctor.html/.
As with any medical treatment, there are some risks which include painful or frequent urination, blood in the urine or semen, decrease in ejaculatory volume, urinary tract infection (UTI), inability to urinate or completely empty the bladder and urgent need to urinate. Visit Rezum.com for a complete list of risks and talk to your doctor about the benefits and risks associated with Rezūm.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
All treatments have inherent and associated risks. The Rezūm System is intended to relieve symptoms, obstructions and reduce prostate tissue associated with BPH. It is indicated for men ≥ 50 years of age with a prostate volume 30cm3 ≤ 80cm3. The Rezūm System is also indicated for treatment of prostate with hyperplasia of the central zone and/or a median lobe. Potential risks include but are not limited to painful urination (dysuria), blood in the urine (hematuria), blood in the semen (hematospermia), decrease in ejaculatory volume, suspected urinary tract infection (UTI), and urinary frequency, retention or urgency. You should talk with your doctor about benefits and risks before moving forward with any treatment option.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.
References
1. Harvard Health. The growing problem of an enlarged prostate gland. Available at: https://www.health.harvard.edu/mens-health/the-growing-problem-of-an-enlarged-prostate-gland. Accessed December 2024.
2. Health Information. Urologic Diseases. Prostate Problems. Prostate Enlargement (Benign Prostatic Hyperplasia). National Institute of Diabetes and Digestive and Kidney Diseases. Available at: https://www.niddk.nih.gov/health-information/urologic-diseases/prostate-problems/prostate-enlargement-benign-prostatic-hyperplasia#benign. Accessed December 2024.
3. Diseases & Conditions. Symptoms & causes. Benign prostatic hyperplasia (BPH). Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/symptoms-causes/syc-20370087. Accessed December 2024.
4. UpToDate. Patient education: Benign prostatic hyperplasia (BPH) (Beyond the Basics). Available at: https://www.uptodate.com/contents/benign-prostatic-hyperplasia-bph-beyond-the-basics. Accessed December 2024.
5. Diseases & Conditions. Diagnosis & treatment. Benign prostatic hyperplasia (BPH). Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/diagnosis-treatment/drc-20370093. Accessed December 2024.
6. McVary KT, Gittelman MC, Goldberg KA, et al. Final 5-year outcomes of the multicenter randomized sham-controlled trial of Rezūm water vapor thermal therapy for treatment of moderate-to-severe lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2021 Sep;206(3):715-24.
7. McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation: A multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016 May;195(5):1529-38.
Get the Facts about Enlarged Prostate
(BPT) – Sponsored by Boston Scientific
Enlarged prostate, also known as benign prostatic hyperplasia (BPH), is a common condition that affects about 50% of men by age 60 and up to 90% of men by age 85. 1 As men age, the prostate can grow from the size of a walnut to about the size of a lemon, placing pressure on the urethra and obstructing the flow of urine. 1 While enlarged prostate (BPH) can be a burdensome condition, it is non-cancerous and there are several treatment options available. 2
What are the early signs of enlarged prostate and common symptoms?
Symptoms of enlarged prostate vary and tend to worsen over time. Common symptoms of enlarged prostate include frequent and urgent urination, trouble starting a urine stream, urinary retention, urinary incontinence and more. 2 Interestingly, the size of the prostate doesn’t always determine how serious symptoms are. For instance, some men with slightly enlarged prostates can experience major symptoms, while others with very enlarged prostates can experience minor symptoms. 3 Less frequent but serious symptoms to watch out for include urinary tract infection, not being able to pee and blood in the urine. 3
Is there a way to treat enlarged prostate? What are some common treatment options?
It is important to discuss treatment options with a health care provider as soon as symptoms begin. 3 While there isn’t a cure for enlarged prostate, a range of treatment approaches can help alleviate symptoms, including:
• Watch and wait: Closely monitoring the condition is the first consideration for most men presenting with early signs of enlarged prostate. Some men will also turn to behavioral modifications such as drinking less caffeine to avoid worsening symptoms. 4
• Medication: Another way of treating enlarged prostate is through commonly prescribed medications that can relax the tension on your urethra making it easier to urinate. Other medications block hormones, which in turn can slow the growth of the prostate gland. 5
• Surgery: In instances where medications are ineffective or patients wish to avoid daily pills and their associated side effects, symptoms are severe or complications arise, health care providers may recommend surgery to remove the tissue around the urethra or widen the urethra itself. 5
Are there alternatives to surgery?
For those who want to avoid surgery, there are minimally-invasive treatment options, like Rezūm™ Water Vapor Therapy — a short, in-office procedure requiring no general anesthesia. 6 The procedure uses water vapor, or steam, to remove obstructive prostate tissue over time — helping reduce the symptoms of BPH. 7
Most patients return to their regular activities within a few days and see improvement in their symptoms over a few weeks. Research has also shown that Rezūm provides patients with significant and sustained BPH symptom relief through 5 years. 6
Graphic depicts how Rezūm Water Vapor Therapy affects the prostate.
To learn more about Rezūm Water Vapor Therapy and to find a doctor near you, please visit https://www.rezum.com/find-a-doctor.html/.
As with any medical treatment, there are some risks which include painful or frequent urination, blood in the urine or semen, decrease in ejaculatory volume, urinary tract infection (UTI), inability to urinate or completely empty the bladder and urgent need to urinate. Visit Rezum.com for a complete list of risks and talk to your doctor about the benefits and risks associated with Rezūm.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
All treatments have inherent and associated risks. The Rezūm System is intended to relieve symptoms, obstructions and reduce prostate tissue associated with BPH. It is indicated for men ≥ 50 years of age with a prostate volume 30cm3 ≤ 80cm3. The Rezūm System is also indicated for treatment of prostate with hyperplasia of the central zone and/or a median lobe. Potential risks include but are not limited to painful urination (dysuria), blood in the urine (hematuria), blood in the semen (hematospermia), decrease in ejaculatory volume, suspected urinary tract infection (UTI), and urinary frequency, retention or urgency. You should talk with your doctor about benefits and risks before moving forward with any treatment option.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.
References
1. Harvard Health. The growing problem of an enlarged prostate gland. Available at: https://www.health.harvard.edu/mens-health/the-growing-problem-of-an-enlarged-prostate-gland. Accessed December 2024.
2. Health Information. Urologic Diseases. Prostate Problems. Prostate Enlargement (Benign Prostatic Hyperplasia). National Institute of Diabetes and Digestive and Kidney Diseases. Available at: https://www.niddk.nih.gov/health-information/urologic-diseases/prostate-problems/prostate-enlargement-benign-prostatic-hyperplasia#benign. Accessed December 2024.
3. Diseases & Conditions. Symptoms & causes. Benign prostatic hyperplasia (BPH). Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/symptoms-causes/syc-20370087. Accessed December 2024.
4. UpToDate. Patient education: Benign prostatic hyperplasia (BPH) (Beyond the Basics). Available at: https://www.uptodate.com/contents/benign-prostatic-hyperplasia-bph-beyond-the-basics. Accessed December 2024.
5. Diseases & Conditions. Diagnosis & treatment. Benign prostatic hyperplasia (BPH). Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/diagnosis-treatment/drc-20370093. Accessed December 2024.
6. McVary KT, Gittelman MC, Goldberg KA, et al. Final 5-year outcomes of the multicenter randomized sham-controlled trial of Rezūm water vapor thermal therapy for treatment of moderate-to-severe lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2021 Sep;206(3):715-24.
7. McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation: A multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016 May;195(5):1529-38.