2026-02-11T08:09:00
(BPT) – During the colder months, people often feel stiff, sluggish or vaguely “off” compared to other seasons, making it difficult to move comfortably and maintain a steady routine. This winter slump can become even more challenging as you age. However, winter stiffness and fatigue aren’t just about “slowing down.”
They’re often signs of increased inflammation.
Inflammation affects how you move, recover and stay consistent. It’s also often an underlying cause of seasonal discomfort and reduced mobility. And when inflammation goes unaddressed, it can stifle your ability to stay active, productive and independent as you age.
“The good news is that inflammation is often manageable,” said Dr. Dustin DebRoy, DC, manager of education and relations at The Joint Chiropractic. “By focusing on small, supportive lifestyle adjustments, people can reduce inflammation and reset their body this winter and — more importantly — their long-term health.”
Small changes with big impact
Major short-term fixes can’t sustain long-term performance, mobility and healthy aging. Instead of committing to a complete life overhaul, focus on building foundational habits that help you manage inflammation so you can improve how your body feels and moves.
Here are just a few small lifestyle adjustments that can have a big impact on inflammation, allowing your body to rest and reset.
Eat an anti-inflammatory diet: What you feed your body can also feed your inflammation. According to Harvard Health, refined carbohydrates, fried foods, sweet beverages, red meat, processed meat and unhealthy fats can increase inflammation. Try to limit eating these types of food and instead incorporate anti-inflammatory options like fruits and vegetables, whole grains, lean proteins and healthy fats like olive oil.
Manage stress: Stress can take a toll on your entire body and directly contributes to inflammation. According to The Joint Chiropractic, stress elevates your cortisol levels, setting off your body’s natural inflammatory response. Make time in your day to unwind by meditating, journaling, walking or doing other relaxing activities. Not only do these activities help you destress and manage your inflammation, but they also can create long-term habits that may help promote calmness, mobility and overall well-being.
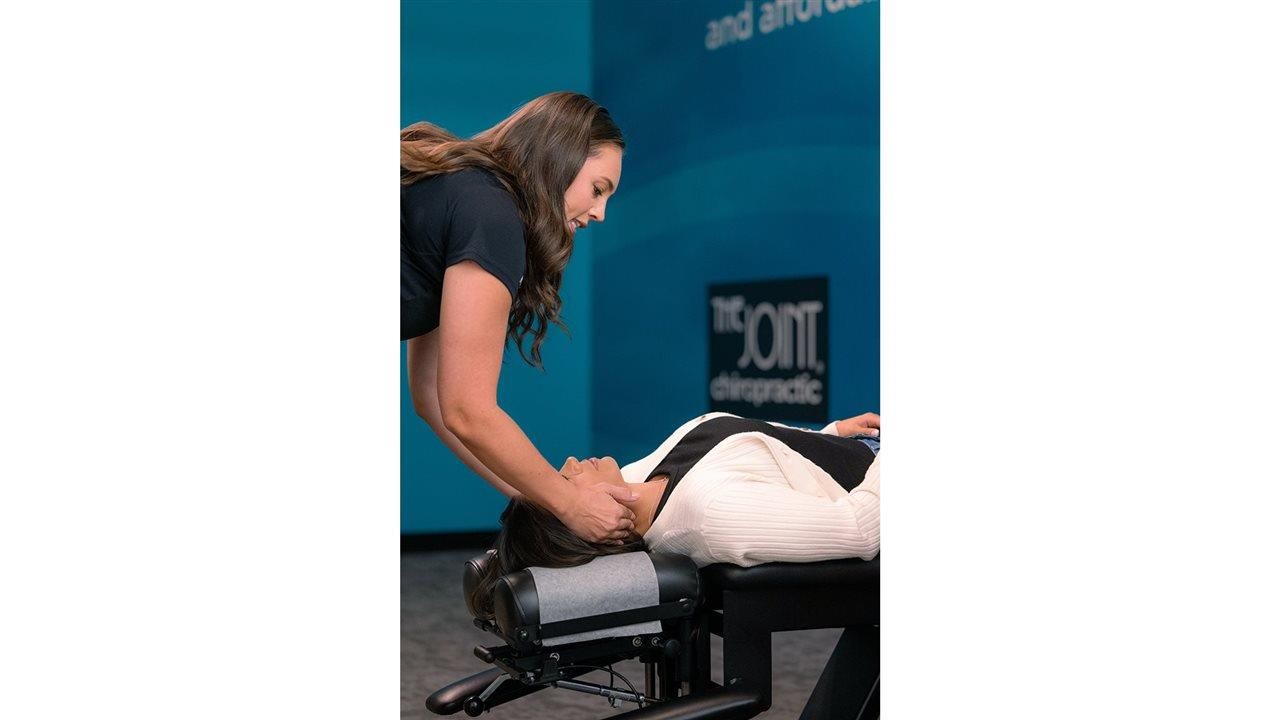
Schedule chiropractic care: Your body’s musculoskeletal alignment affects how you move and feel. When you’re out of alignment, you may feel discomfort and stiffness, making it harder to move. By visiting a chiropractic professional and receiving regular adjustments, you may feel reduced pain and stiffness in your joints, improved mobility, reduced stress and increased blood flow, all of which can contribute to a more active lifestyle and reduced inflammation. To learn more about how chiropractic care may help and to find a chiropractic professional near you, visit TheJoint.com.
Take a few minutes to stretch: Speaking of bodily alignment, stretching is another way to support your body so you can move more with less pain. You don’t need to spend a long time doing so. In as little as six minutes, you can improve your flexibility, relieve joint stiffness and reduce stress.
Realign, reset and recover in 2026 and beyond
Small, sustainable changes are the key to managing inflammation and unlocking a better-feeling you. This winter, start small and build on these and other healthy habits that will help your body realign, reset and recover not just this season, but for the rest of your life.